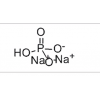
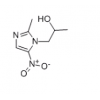
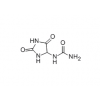
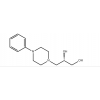
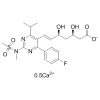
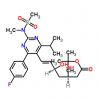
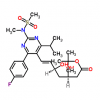
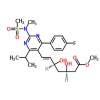
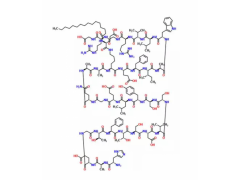
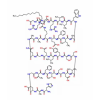
Synonym:Liraglutide;Glycine, L-histidyl-L-alanyl-L-alpha-glutamylglycyl-L-threonyl-L-phenylalanyl-L-threonyl-L-seryl-L-alpha-aspartyl-L-valyl-L-seryl-L-seryl-L-tyrosyl-L-leucyl-L-alpha-glutamylglycyl-L-glutaminyl-L-alanyl-L-alanyl-N6-(N-(1-oxohexadecyl)-L-gamma-glutamyl)-L-lysyl-L-alpha-glutamyl-L-phenylalanyl-L-isoleucyl-L-alanyl-L-tryptophyl-L-leucyl-L-valyl-L-arginylglycyl-L-arginyl-;Liraglutida;Liraglutida [inn-spanish];Liraglutide [usan:inn];Liraglutidum;Liraglutidum [inn-latin];N26-(Hexadecanoyl-gamma-glutamyle)-(34-arginine)glp-1-(7-37)-peptide
MF:C172H265N43O51
MW:3751.202
Product Category: GLP
Pharmacodynamic and Pharmacology Properties: Liraglutide is an acylated GLP-1 analogue that shares 97 % amino acid sequence homology to human endogenous GLP-1 (7–37). The single amino acid substitution of lysine with arginine at position 34 and the attachment of a C16 fatty acid chain to lysine at position 26 enables liraglutide to self-associate and form a heptameric structure, which delays absorption from the subcutaneous injection site and provides protection against degradation by DPP-4 enzyme and neutral endopeptidases. As a consequence, liraglutide has a much longer half-life than endogenous GLP-1 (&13 h vs. 1.5–2 min).
Liraglutide binds to and activates the GLP-1 receptor, which is a membrane bound cell-surface receptor coupled to adenyl cyclase by the stimulatory G-protein (Gs) in pancreatic b cells (but not in pancreatic a-cells) and is the target for endogenous GLP-1. This results in increased intracellular cyclic monophosphate and subsequent liraglutide dose-dependent insulin release in patients with elevated glucose levels. At the same time, liraglutide acts in a glucose-dependent manner to decrease inappropriately high glucagon secretion, thereby blocking the effects of glucagon on hepatic glucose output. In the presence of liraglutide, as blood glucose concentrations decrease, the secretion of insulin diminishes and blood glucose concentrations approach euglycaemia. In addition to these glucoregulatory mechanisms of action in patients with type 2 diabetes, liraglutide slightly delays gastric emptying, and reduces bodyweight and body fat mass by reducing hunger and lowering energy intake; these effects may contribute to the beneficial effects of liraglutide in patients with type 2-diabetes. As reviewed previously, in patients with type 2 diabetes and/or in preclinical studies, liraglutide dosedependently reduced glycated haemoglobin (HbA1c), fasting plasma glucose (FPG) and postprandial plasma glucose levels, with sustained improvements in glucose levels over a 24-h dosage interval. In addition, liraglutide increased insulin secretion, reduced postprandial glucagon secretion, improved surrogate measures of b-cell function, reduced systolic blood pressure (SBP) and improved some biomarkers of cardiovascular (CV) risk in patients with type 2 diabetes, including suppression of postprandial triglyceride and apolipoprotein B48 levels after a fat-rich meal. Improvements in glycaemic control and other efficacy outcomes in adult patients with type 2 diabetes receiving liraglutide monotherapy or add-on therapies to other antidiabetic drugs in large phase III trials.
Description: Approaches to treating T2DM, a disease characterized by the dual defect of islet cell dysfunction and insulin resistance, include agents that increase the secretion of insulin by the pancreas (secretagogues), agents that increase the sensitivity of target organs to insulin (sensitizers), and agents that decrease the glucose absorption rate from the gastrointestinal tract.Liraglutide, the GLP-1 receptor agonist to reach the market, possesses a 97% homology to GLP-1 with only two amino acid changes and the addition of a fatty acid side chain. Specifically, the lysine in position 34 has been replaced with an arginine, and the lysine in position 26 has been modified with a C16 acyl chain via a glutamoyl spacer. Liraglutide derives its resistance to DPP-4 degradation from its propensity to form micelles and to bind to albumin. Unlike its predecessor exenatide, which requires two daily subcutaneous injections before the first and last meals of the day, liraglutide is approved as a once-daily treatment regimen and may be used in combination with metformin or a sulfonylurea in patients with insufficient glycemic control with either monotherapy or combined dual therapy. It is also approved in combination with the dual therapy of metformin and a thiazolidinedione in patients with insufficient glycemic control. Liraglutide displayed a binding potency of 61 pM (EC50= 55 pM for GLP-1) for the cloned human GLP-1 receptor.



 通过认证
通过认证